
Kuijpers Instruments Groesbeek
Bredeweg 48
6562 DG Groesbeek
info art. bone harvesting KSK-363


Kuijpers Instruments Groesbeek
Bredeweg 48
6562 DG Groesbeek
info art. bone harvesting KSK-363

The instrument complies with the CE mark for Medical Devices, class 1..
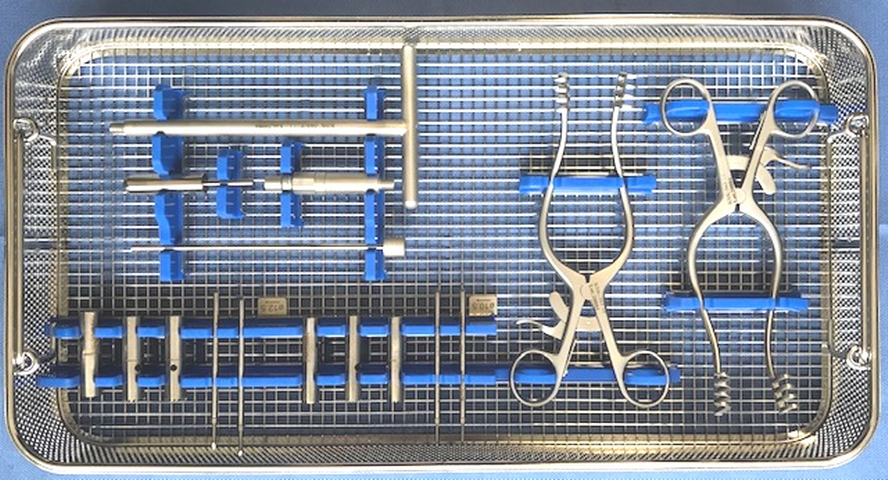
Set description:
autologous (power tool assisted) bone harvesting set to collect bone dowels (plugs) from the iliac crest. No disposable single use parts. One tray set.
Objective:
To collect autologous bone dowels (1-3) of compact cancellous bone from the iliac crest with a power tool and keep the normal contour of the iliac crest while restoring the soft tissue coverage of the iliac crest.
Set contents:
Plug reamers diameters 10.5 and 12.5 mm
Adaptive cannulated reamer connector to battery power tool
Guide wire with stop (2)
Plug cutters diameters 10.5 and 12.5 mm
Adaptive cannulated handle for plug cutter
Impactor to release bone plug from plug cutter
Surgical technique:
Incision (3-5 cm) dorsal and parallel to the iliac crest. Expose periosteum and m. obliquus externus abdominus attachment. Release abdominal musculature attachment to ventral to expose the iliac crest. Keep m. gluteus medius attachment intact. Remove periosteum from iliac crest. Use self-retaining retractor.
Introduce guide wire in direction of iliac crest bone anatomy. Use plug reamer of appropriate size attached to battery power tool and ream until stop. Remove reamer and exchange for manual handle and plug cutter. Twist 90 and take plug holder and bone plug out. Fill hole with TriCalciumPhosphate (Synthes) resorbable plug of appropriate size.
Break of plug and restore bony contour of iliac crest.
Repeat for more bone plugs.
Hemostasis. No drain. Wound closure in layers. Re-attach m. obliquus externus abdominus covering the iliac crest bone surface. Close subcutaneous layer. Close skin. Infiltrate with local anesthetic Ropivacaine.
The manufacturer supplies a product with an article number from the company, the product has been checked for sharpness, finish, efficiency, the data are recorded as material choice lot no. Article number and CE no. The product is packaged and supplied is then sterilization data and the product is released on delivery. The user The user must perform a visual inspection when taking / receiving the product. After a visual inspection, the user must, if possible, inform the supplier whether the product has been approved. In the event of insufficient performance or faults, the user must contact the manufacturer who then checks the product and, if necessary, takes measures.
The instrument is delivered disinfected, as a first step it is disinfected with Allclean acc. VAH and European standards. Before use, you must remove the packaging and visually inspect it for defects or damage. As a final step, you should carry out the high-level disinfection / sterilization in the packaging you want to prepare immediately for surgical use.
For each subsequent use, it is necessary to check for damage and defects, deformations and a visual check, and to check the instruments for sharpness and operation.
The instrument may be cleaned by machine.
Maintenance instructions.
Maintenance is not necessary
What to do with complaints
If an instrument no longer satisfies the requirements, or in the event of defects or damage, contact the manufacturer who will subject it to an accurate inspection and performs sinful repairs and / or takes measures.
T-Handle KIG-363-T
Emissions pin KIG-363-U
Driver handpiece KIG-363-D
Key KIG-363-K
Fixation pen 9,5 KIG-363-Fp. 2X
Fixation pen12,5 KIG-363-Fp. 2X
Mill 12,5 KIG-363-12,5 2x
Mill 9,5 KIG-363-10,5 2x
Cracker 12,5 KIG-363-12,5-C
Cracker 9,5 KIG-363-10,5-C
Spreaders Le+Re KIG-363-S